Atomic Force Microscopy (AFM) for Optimization of Silica Chemical Inhibition in Geothermal Brines
- 07 Oct 2015
- Volume 5
- NanoScientific Magazine, Fall 2015


Silica scaling has been a huge problem for geothermal plants because it significantly lowers the efficiency of heat extraction from geothermal fluids. Geothermal electricity is generated by using the geothermal energy to rotate the turbine that activates the generator. In contrast with most power plants that use fossil fuels, geothermal power plants utilize the steam produced from the water reservoir found a couple of miles below the earth’s surface (Figure 1).
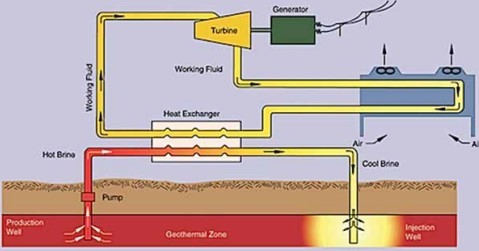
Figure 1. Schematic diagram of a geothermal power plant (credit www.seg.gov)
The extracted geothermal fluid usually contains minerals that can lead to scaling. The presence and level of silication on pipes, heat exchanges, storage tanks, etc. in the extracted geothermal fluid is one of the major parameters in the design and operation of the plant. Silica is known to polymerize and precipitate from water soluble monomeric silica when it exceeds the saturation level. This could be due to the decrease in pressure or temperature of circulating fluids. The deposition of the silica on the pipe and instrument walls increases the resistance to heat flow and therefore the amount of heat extracted from the geothermal fluid. Extreme cases of silica scaling also constricts the flow, which further decreases the efficiency of heat extraction (Figure 2).

Figure 2. Scaling and Silica deposition on pipes handling geothermal brine (credit www.geokem.co.nz)
The amount of silica in the geothermal fluid is currently being monitored by performing silicomolybdate test (ASTM D859-00), which measures the amount of oligomeric silica in the geothermal fluid. The silicomolybdate test involves the formation of the yellow color due to the reaction between the molydate ion with silica and phosphate under acid condition. If the concentration of silica is low, then amino-naphthol sulfonic acid can be added to convert the faint yellow to dark blue. Although the silicomolybdate test can be used to check if the silica level is going beyond the saturation level, it fails to see any premature silica polymerization brought about by sudden change in chemistry of the geothermal fluid. Other disadvantage includes interferences from substances that are present in the geothermal fluid such as iron compounds. Large amounts of ferrous and ferric substances can be introduced to the geothermal fluid due to the corrosion of the pipes and equipment. Other interferences can come from phosphate, slowreacting forms of silica, sulfides, and turbidity. Unfortunately, all of these are usually present in geothermal fluids. Therefore, extra care should be done in interpreting the results of the silicomolybdate test if used to geothermal fluids.
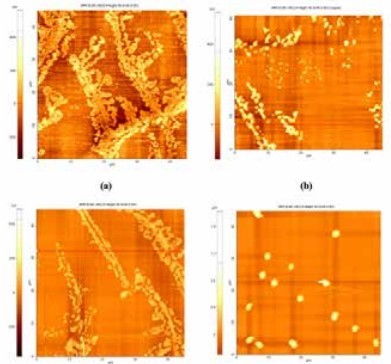
Figure 3. AFM images of the silica polymerized from (a) untreated geothermal fluid, (b) 5.0 pm, (c) 8 ppm, (d) 10 ppm of phosphinocarboxylic acid.

Figure 4. Atomic force microscopy system (Courtesy Park Systems Inc).
Figure 4. Atomic Force Microscopy (AFM) can be used to effectively monitor the presence of the polymeric silica nanoparticles in the geothermal fluid. The AFM is coupled with the silicomolybdate test to have a complete picture of the silica polymerization process. Basically, it measures the amount of silica still dissolved in solution, while the AFM measures the size of the polymerized silica particles. The combined technique is used to determine the optimum dosage of the phosphinocarboxylic acid, a known silica inhibitor.The solution containing the polymerized silica particles was dropcasted on freshly prepared mica substrate. Excess solution was wicked away and the mica substrate with the silica nanoparticles was dried for at least 24 hours. Park NX10AFM from Park Systems in non-contact mode was used to image and measure the particle size of the polymerized silica nanoparticles. In non-contact mode, the cantilever is driven to oscillate at its resonance frequency. This imaging mode lessens the chance to move the silica particles during scanning. Figure 3. shows the silica polymerized from geothermal solution containing different levels of the phosphinocarboxylic acid inhibitor. One can see from the figure that the particle size decreases as we add more silica inhibitor. However, adding too much actually worsens the problem because it actually promotes the formation of larger silica particles. It can also be seen that addition of the phosphinocarboxylic acid inhibitor decreases the degree of linking among the precipitated silica particles. At 10 ppm dosage, it can be seen that silica are present as isolated particles on mica. In conclusion, the AFM proved to be a very valuable tool in measuring the size of the silica particles polymerized from the geothermal fluid. The AFM coupled with the conventional silicomolybdate test provide a more holistic understanding of the phenomena of scaling even before it starts significantly affecting the efficiency of heat extraction from geothermal fluid.
